Expertise.
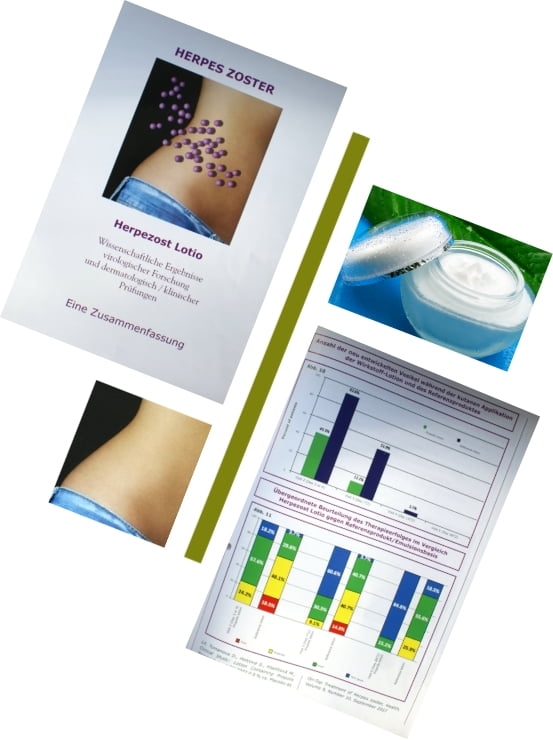
Scientific studies and regulations.
The high requirements of the ministries of health and the international licensing authorities ensure safety. Gehrlicher produces scientific data while taking these requirements into account,
initiates and supervises studies in pharmacology, and both clinical and practical studies, in order to test and confirm the efficacy of the phyto-active ingredients.
Our wealth of experience gained over 50 years is a solid basis for the implementation of theoretical considerations, experimental results from the laboratory, pharmacology and clinical practice.
initiates and supervises studies in pharmacology, and both clinical and practical studies, in order to test and confirm the efficacy of the phyto-active ingredients.
Our wealth of experience gained over 50 years is a solid basis for the implementation of theoretical considerations, experimental results from the laboratory, pharmacology and clinical practice.
Active substance "Nature".
New methods for the qualitative and quantitative detection of active or lead substances are constantly being developed in the analytical and pharmaceutical field. Validations are carried out and new methods are used in order to gain reliable information about the quality of an extract.
Stability studies are as much a focus of our work as the validation of tried-and-tested analytical methods.
Stability studies are as much a focus of our work as the validation of tried-and-tested analytical methods.

Natural product research.
Range of services for clinical trials.
Gehrlicher maintains very close cooperation with scientific institutes, university clinics, medical practices, private scientific institutions and laboratories.
The separate GMP-compliant department for the manufacture
of finished medications also guarantees the manufacture of test samples and placebos, which means that clinical trials that comply with the ICH Guideline
can be carried out in the clinics and in medical practice. Significant results have already been achieved in clinical trials for the development and generation of new products.
These trials result in numerous scientific publications.
The separate GMP-compliant department for the manufacture
of finished medications also guarantees the manufacture of test samples and placebos, which means that clinical trials that comply with the ICH Guideline
can be carried out in the clinics and in medical practice. Significant results have already been achieved in clinical trials for the development and generation of new products.
These trials result in numerous scientific publications.
Clinical and scientific research is the priority.